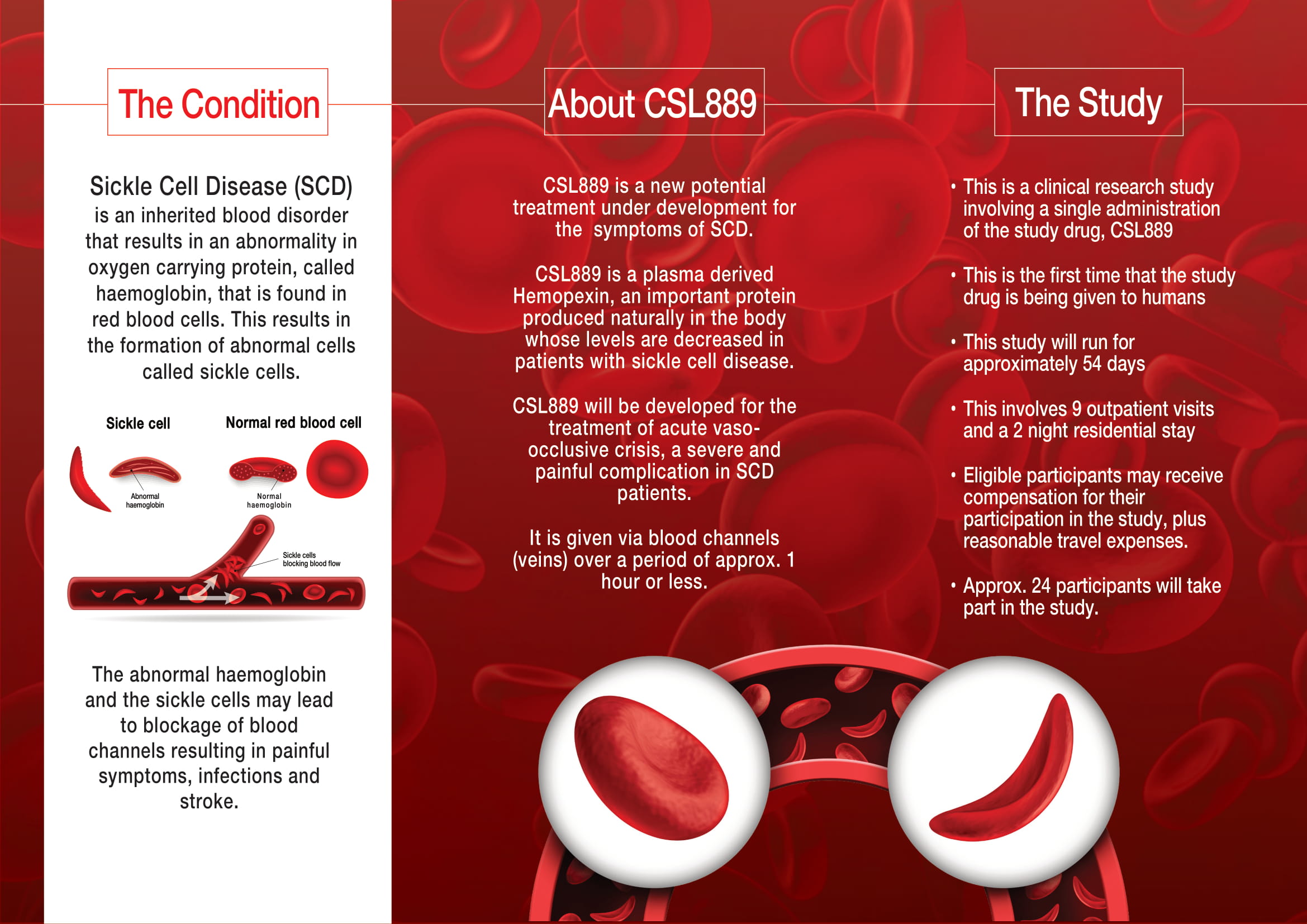
CSL889 Clinical Trial
About CSL889
CSL889 is a new potential treatment under development for the symptoms of SCD.
CSL889 is a plasma derived Hemopexin, an important protein produced naturally in the body whose levels are decreased in patients with SCD.
CSL889 will be developed for the treatment of acute vasoocclusive crisis, a severe and painful complication in SCD patients.
It is given via blood channels (veins) over a period of approx. 1 hour or less.
The Study
- This is a clinical research study involving a single administration of the study drug, CSL889
- This is the first time that the study drug is being given to humans.
- This study will run for approximately 54 days
- This involves 9 outpatient visits and a 2 night residential stay
- Eligible participants may receive compensation for their participation in the study, plus reasonable travel expenses.
- Approx. 24 participants will take part in the study
Currently Open Clinical Trial Locations:
- King’s College Hospital – Denmark Hill London SE5 9RS
- Guy’s & St Thomas Hospital – Great Maze Pond London SE1 9RT
- UCLH – 235 Euston Road London NW1 2BU
- Manchester Royal Infirmary – Oxford Road, Manchester M13 9WL. Contact Number: 0161 906 7510
- MAC’s Early Phase Unit – Nelson Street Manchester M13 9NQ
- Free phone 0800 917 7637
- Link to register for the trial: https://bit.ly/3wCNx8A
If you are interested in this trial, you can also speak to your treating physicians who can get in touch with the participating sites on your behalf.